What is the niPOC test?
niPOC is an advanced non-invasive test which uses a blood sample to investigate whether a pregnancy loss may have been caused by a chromosome abnormality, by analysing circulating foetal DNA.
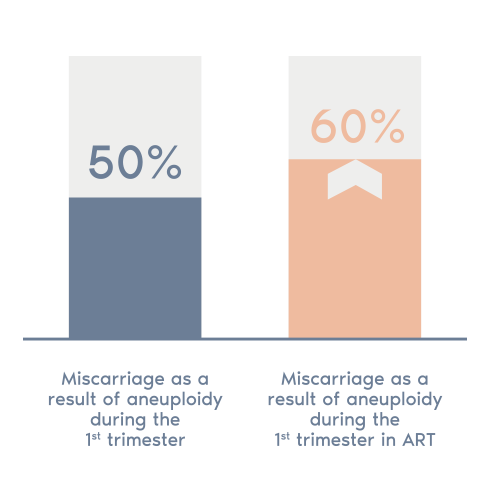
- In around 50% of first trimester miscarriages a chromosome abnormality is present
- This figure rises to 60% in the case of women who receive assisted reproductive treatment and further increases with age.
- 24 chromosomes are screened to identify the reason for the miscarriage.
- The information provided by the niPOC test may reduce the likelihood of another miscarriage and help patients make future decisions about their reproductive health.

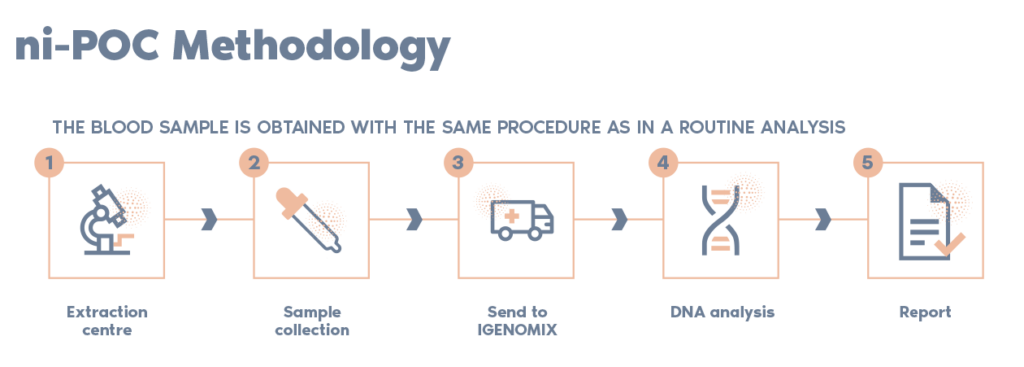
**It is essential that the blood sample is collected before the evacuation of the foetal tissue takes place. If pharmacological treatment is being used for the evacuation, the blood should be collected prior to taking the medication.